Iron Reactive Barriers at the Oak Ridge Y-12 Site
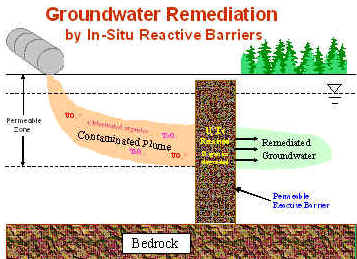 The concept |
 Barrier construction |
Two permeable iron reactive barriers (a continuous trench and a funnel-and-gate system) were installed in late November, 1997 at the U.S. Department of Energy’s Y-12 National Security Complex in Oak Ridge, Tennessee. The overall goal of this research was to determine the effectiveness of the use of zero-valent iron (Fe0) to retain or remove uranium and other contaminants such as technetium and nitrate in groundwater. The long-term performance issues were investigated by studying the biogeochemical interactions between Fe0 and groundwater constituents and the mineralogical and biological characteristics over an extended field operation.6 Results from up to 8 years of monitoring indicated that the Fe0 was effective in removing contaminant radionuclides such as uranium and technetium.2, 3 In addition, a number of groundwater constituents such as bicarbonates, nitrate, and sulfate were found to react with the Fe0. Both nitrate and sulfate were reduced within or in the influence zone of the Fe0 where redox potential was low.6, 10 An increased anaerobic microbial biomass was also observed within and in the vicinity of the Fe0 barrier, and these microorganisms were at least partially responsible for the reduction of nitrate and sulfate in groundwater.4 Decreased concentrations of Ca2+ and bicarbonate in groundwater occurred as a result of the formation of minerals such as aragonite (CaCO3) and siderite (FeCO3), which coincided with the Fe0 corrosion and an increase of groundwater pH.7, 9, 11 A suite of mineral precipitates was identified in the Fe0 barrier, including amorphous iron oxyhydroxides, goethite, ferrous carbonates and sulfides, aragonite, and green rusts. These minerals were found to be responsible for the cementation and possibly clogging observed in a number of core samples from the barrier. Significant cementation progressed as the operation of the iron barrier and it corresponded to the changes in a decrease in hydraulic gradient and connectivity.9, 10 The study concludes that, while Fe0 may be used as an effective reactive medium for the retention or degradation of many redox-sensitive contaminants, its long-term reactivity and performance could be severely hindered by its reactions with other groundwater constituents; and groundwater flow may be restricted because of the build-up of gases12 and mineral precipitates at the soil/Fe0 interface.11 Depending on the site biogeochemical conditions, the rate of Fe0 corrosion may increase; therefore seriously shortens the life span of the Fe0 barrier that was predicted previously (~15–30 years).
References:
- Liang, L, NE Korte, JD Goodlaxson, J Clausen, Q Fernando, R Muftikian. 1997. Byproduct formation during reduction of TCE by zero-valence iron and palladized iron. Ground Water Monitoring & Review, Winter:122-127.
- Gu, B., L Liang, MJ Dickey and S Dai. 1998. Reductive precipitation of uranium (VI) by zero-valence iron. Environ. Sci. Technol., 32:3366-3373.
- Birke, V, L Liang, H Burmeiser. 1999. Durchströmte reaktoren zur In-situ grundwassersanierung. TerraTech, 1999 *1):23-25.
- Gu, B, TJ Phelps, L Liang, MJ Dickey, BL Kinsall, and GK Jacobs. 1999. Biogeochemical dynamics in zero-valent iron columns: implications for permeable reactive barriers. Environ Sci & Technol 33:2170-2177.
- Korte, NE, JL Zutman, RM Schlosser, L Liang and Q Fernando. 2000. Field application of palladized iron for the dechlorination of trichloroethene. Waste Management 20:687-694.
- Liang, L, N Korte, B Gu, R Puls, C Reeter. 2000. Geochemical and microbial reactions affecting the long-term performance of In Situ iron barriers. Advances in Environ Res 4: 273-286.
- Phillips, DH, B Gu, DB Watson, Y Roh, L Liang, and SY Lee. 2000. Performance evaluation of a zero-valent iron reactive barrier: mineralogical characteristics. Environ Sci Technol 34:4169-4176.
- Korte, N, OR West, L Liang, B Gu, JL Zutman and Q Fernando. 2002. The effect of solvent concentration on the use of palladized-iron for the step-wise dechloriation of polychlorinated biphenyls in soil extracts. Waste Management 22:343-349.
- Kamolpornwijit, W, L Liang, OR West, GR Moline, AB Sullivan. 2003. Heterogeneity development and its influence on long-term PRB performance: a column study. J Contaminant Hydrology, 66:161-178.
- Liang, L, AB Sullivan, OR West, W Kamolpornwijit, GR Moline. 2003. Predicting the precipitation of mineral phases in permeable reactive barriers. Environ Eng Sci 20(6):635-653.
- Kamolpornwijit, W, L Liang, GR Moline, T Harts, and OR West. 2004. Identification and quantification of mineral precipitates in Feº fillings from a column study. Environ Sci Technology 38:5757-5765.
- Kamolpornwijit, W and L Liang. 2006. Investigation of gas production and entrapment in granular iron medium. J Cont Hydrology 82:338-356.

