Uranium(VI) Removal by Zero-Valent Iron Metal
|
At the Y-12 site, uranium is the main contaminant for this groundwater remediation using the permeable Fe0 reactive barrier because uranium poses the major potential health and environmental risks within Bear Creek Valley. Its concentration trends within and in the vicinity of the Fe0 barrier are plotted in Figure 1 for some monitoring wells and piezometers. Results indicate that uranium is effectively removed within the Fe0 barrier, and that uranium concentrations in the Fe0 barrier (e.g., TMW-09, DP-19s,m, and DP-20s,m) were generally very low (<0.01 mg/L) in comparison with uranium concentrations in the upgradient monitoring wells. With exception of DP-11, low amounts of uranium were also found in the downgradient monitoring wells (e.g., DP-23s,m, and DP-14s, TMW-7), suggesting that the Fe0 barrier is performing well in removing uranium from the contaminated groundwater. Similarly, uranium concentrations in other monitoring wells and piezometers (such as DP-10, DP-18s,m,d; DP-21s,m; DP-22s) were found to be low or below the detection limit in the Fe0 barrier (data not shown). These observations are generally consistent with previous laboratory studies that show uranium can be effectively and rapidly reduced by Fe0 filings (Gu et al., 1998; Birke et al., 1999). However, a consistently high uranium was detected in the downgradient DP-11 well, and its concentration was even higher than those found in the upgradient monitoring wells. This observation was primarily attributed to the fact that some uranium hot spots existed in such heterogeneous filled materials as evidenced by our recent soil core sampling and analysis near DP-11 (conducted in September 2001). Another possible explanation was due to an upward flow of the contaminated groundwater as result of the vertical hydraulic gradient.
Uranium retention by Fe0 filings was also evidenced by the analysis of uranium content in Fe0 core samples (Figure 2). These angled cores were collected ~15 months after installation of the Fe0 barrier, and detailed sample preparation and analysis were given elsewhere (Phillips et al., 2000). Although only trace quantities of uranium are present in the contaminated groundwater, an elevated amount of uranium was detected in the Fe0 core materials, particularly in those samples near the interface (between the soil and Fe0 filings) where groundwater enters the Fe0 barrier as seen in column studies (Kamolpornwijit et al., 2003). The greatest concentration of uranium in the Fe0 barrier occurred at the shallow, upgradient interface, but uranium concentration decreased dramatically over a short distance. These observations suggest that once uranium enters the Fe0 reactive barrier it is rapidly sequestered in situ as a result of either reductive precipitation of relatively insoluble U(IV) species or surface adsorption of U(VI) species on the Fe0 corrosion products (e.g., iron oxyhydroxides) (Cantrell et al., 1995; Gu et al., 1998). Reductive precipitation of U(VI) to U(IV) species by Fe0 is believed to be one of the dominant mechanisms for uranium removal and is thermodynamically favorable, according to the following stoichiometric reactions :
Fe2+ + 2e- ===> Fe(0) e0 = -0.440 V (1)
UO22+ + 4H+ + 2e- ===> U(IV) + 2H2O e0 = +0.327 V (2)
or e = -0.07 V at pH 8.
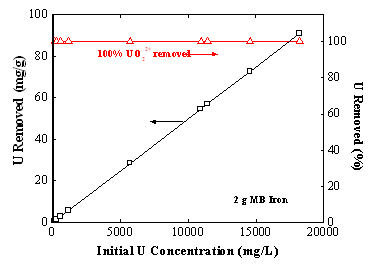
The reduced U(IV) readily forms oxyhydroxide precipitates in aqueous solution. Such a reductive reaction mechanism has been evaluated by laboratory batch equilibrium studies and by sensitive fluorescence spectroscopic analysis (Gu et al., 1998; Birke et al., 1999). The fluorescence spectra gave direct evidence of U(VI) reduction by Fe0 because the reduced U(IV) species do not fluorescence. The batch equilibrium studies provided additional evidence because, regardless of the initial added uranium concentration in solution (up to 20,000 mg/L), no detectable amounts of uranium were found in the equilibrium solutions after reaction with Fe0. These results are indicative of reductive precipitation process rather than a simple sorption process, in which uranyl distributes or partitions between the solution and solid phases depending on the adsorption affinity and capacity on the adsorbent surfaces. On the other hand, sorption by iron oxides and other adsorbent materials was found to be much less effective than Fe0 filings in removing uranium from the solution. A much higher equilibrium U(VI) concentration was observed in samples treated with these materials than in those treated with Fe0 filings. Using the X-ray photoelectron spectroscopy (XPS) technique, Fiedor et al. (1998) also reported that U(VI) was readily reduced to U(IV) species (~75%) by reacting with an Fe0 coupon under anaerobic conditions, although a large solution to Fe0 ratio was used in these laboratory studies.
However, as the corrosion products of Fe0, such as iron oxyhydroxides, accumulate on Fe0 surfaces, uranium removal through sorption or co-precipitation could not be ruled out (Fiedor et al., 1998; Gu et al., 1998; Hsi and Langmuir, 1985; Morrison et al., 1995). Unfortunately, because of a relatively low amount of uranium retained by the Fe0 filings and a possible reoxidation of reduced U(IV) species during sample preparation and extraction, no attempts were made to distinguish whether uranium was reductively precipitated or sorbed by iron oxyhydroxides in these Fe0 barrier materials. However, on the basis of previous laboratory studies (Cantrell et al., 1995; Fiedor et al., 1998; Gu et al., 1998), a conclusion can be made that a majority of uranium could have been retained by the reductive precipitation because of a strong reducing environment within the Fe0 barrier (with a high solid to solution ratio). Understanding of U(VI) removal mechanisms through either reductive precipitation or sorption/co-precipitation has important environmental implications because the reduced U(IV) species on Fe0 surfaces could be potentially re-oxidized when it is exposed to the air or dissolved O2 in a matter of a few hours or days (Gu et al., 1998) . Similarly, the sorbed U(VI) species could be desorbed and therefore remobilized as groundwater geochemistry changes.
For more information, contact:
Baohua Gu (gub1@ornl.gov, 865-574-7286)