Perchlorate Treatment and Destruction – Using Highly Selective, Regenerable Anion Exchange Resins
 |
|
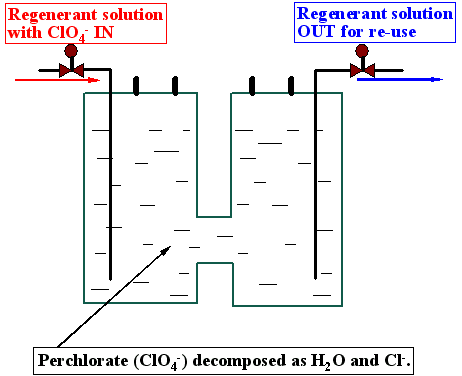
Despite favorable thermodynamics, the high activation energy makes it difficult to degrade perchlorate (ClO4-) at the low concentrations typically encountered in the environment. The Oak Ridge National Laboratory recently developed a new methodology to degrade ClO4- in FeCl3-HCl regenerant solutions using ferrous iron and/or non-toxic organic reducing agents (US Patent 6,358,396). Results indicate that a complete destruction of ClO4- can be achieved in less than 1-h residence time in the FeCl3-HCl solution, and ClO4- ions are decomposed into chloride and water. While ClO4- is reduced, ferrous (Fe2+) ions are oxidized to Fe3+ ions, which replenish or "regenerate" the FeCl3-HCl regenerant solution. Therefore the regenerant solution can be re-used for many cycles, and practically no waste regenerant solution (containing ClO4-) is produced! The destruction of ClO4- can be operated in a flow-through reactor, and a nearly complete degradation of ClO4- is observed under a continuous flow-through mode. Because the FeCl3-HCl solution has been successfully used in regenerating selective anion-exchange resins sorbed with ClO4-, this new methodology offers a cost-effective means to degrade ClO4- while not altering the chemical properties of the FeCl3-HCl regenerant solution, so it can be reused to eliminate the production of secondary wastes.
For more information, contact:
Baohua Gu (gub1@ornl.gov, 865-574-7286)

