Funded Proposal
Problem Statement
DoD faces a potentially daunting task of remediating thousands of metal contaminated sites within the U.S. and its territories which contain unacceptable levels of the toxic metals As(III/V), Cr(III/VI), Cd, and Pb. With the exception of Pb contaminated soils, human health and ecological risk drivers have prompted EPA to assume that the total soil metal concentration is 100% bioavailable. Previous SERDP funded research (CU-1166 and CU-1210), however, has shown that the ubiquitous metal-sequestering properties of soil can significantly lower the bioavailability and risk of heavy metals to human and ecological receptors. Results from CU-1166 show soil components/properties can reduce risk by reducing bioaccessibility of metals upon ingestion relative to the currently used 100% default values. Key soil physical and chemical properties (e.g. particle size, CEC, Fe-oxides, TOC/TIC, pH) were identified as controlling the extent of toxic metal bioaccessibility as measured using an in vitro physiologically-based extraction test (PBET) that simulated the digestive system of humans. Statistical models were developed and incorporated into a predictive tool known as SBAT (Yang et al., 2002 a,b; Stewart et al., 2002 a,b, Heuscher et al., 2004). The bioaccessibility results (in vitro) were found to be in excellent agreement with molecular-level metal speciation studies and in-vivo swine metal bioavailability studies which confirmed that key soil properties control metal bioavailability. Similarly, previous SERDP funded research (CU-1210) has shown that metal-sequestering properties of soil can significantly lower the ecological risk of metal contamination by reducing bioavailability of metals to ecological receptors (e.g. plants, soil invertebrates). Based on these findings, measurement of key soil properties could be used as an indicator tool at DoD sites to determine whether site remediation is necessary or whether more definitive site-specific in vivo metal bioavailability studies are warranted. Nevertheless, site-specific use of the predictive tool is impeded by the lack of regulatory acceptance. This is rational due to the lack of site-specific investigations that couple in vivo bioavailability and in vitro bioaccessibility studies with soil properties and microscopic interrogation of the solid phase metals. Several studies have shown good correlation between the in vitro PBET method and in vivo swine feeding studies for soil Pb, As, and Cd (Ruby et al, 1996; Rodriguez et al., 1999; and Schroder et al., 2003; respectively). However none were designed to investigate DoD site specific soils or considered the role of soil properties in controlling metal bioavailability.
In the following proposed investigation, we seek to bring together regulators, EPA, end-users, and scientists to demonstrate the applicability of these concepts by showing that simple, readily available soil properties can often times be used to predict the bioavailability of As(V), Cd, Cr, and Pb with a reasonable level of confidence. We will show that in vitro methods (i.e., PBET, soil extractions) can be used for risk assessment of toxic metals in soil by comparing in vitro and in vivo metal bioavailability studies. The proposed research is very timely. The use of in vitro methods to determine exposure (i.e., bioaccessibility) to human from soil ingestion has recently gained approval in the UK, and studies funded by the Danish EPA are underway in Denmark this summer to validate a PBET-like in vitro method for accessing bioaccessibility of As, Cd, and Pb in contaminated soils. Other organized research in the EU, Canada, and Australia are conducting similar studies. Several of the researchers in our proposed study are leaders in the area of in vitro gastrointestinal methods to access bioaccessible metals in contaminated soil in the US. The proposed research also is timely in the area of ecological risk assessment. Frameworks to access terrestrial ecological risk assessment (ERA) for assessing risk/cleanup of contaminated soils are under development in the US, EU, Canada, and Australia. USEPA has published an ERA framework that features an eight-step, tiered process. The primary audience for USEPA ERA documentation is risk assessors and managers conducting an ecological risk assessment at contaminated sites (i.e., Superfund sites). The site screening levels will consider the ability of soil properties/components to sequester contaminants and reduce their bioavailability to ecological receptors. Several member of our research team have conducted several research studies funded by USEPA and SERDP (CU-1210) to quantify the effect of soils on contaminant bioavailability for use in the USEPA ERA framework. This research would address the following Navy environmental requirements:
- 1.II.02.d Regulator Approved Methods and Protocols for Conducting Marine and Terrestrial Risk Assessments
- 1.III.01.k Improved Field Analytical Sensors, Toxicity Assays, Methods, and Protocols to Supplement Traditional Sampling and Laboratory Analysis
Research proposed in our study is novel. To our knowledge, our proposed study would be the first field validation study of laboratory in vitro methods to assess the modifying effect of soils on human and ecological risk. The proposed research is timely as has been accomplished in the UK, and soon to be in the EU. We seek to obtain US EPA acceptance of in vitro methods for the assessment of risk associated with soil metal bioavailability. The experimental and numerical endeavors of this proposed work will provide knowledge and information in previously unexplored areas of decreased toxic metal bioavailability in soils to support DoD’s performance/risk assessment and decision-making process for military base site restoration.
Technical Description
Technical Objectives: (1) To provide validation that the relationships between soil properties and in vitro bioaccessibility methods can serve as a screening tool for estimating in vivo toxic metal bioavailability in DoD soils, (2) To provide DoD with a scientifically and technically sound method for estimating human and ecological risk associated with metal contaminated soils and thus the need, or lack there of, for more-detailed, site-specific bioavailability (e.g., animal dosing) studies on DoD lands, and (3) obtain regulatory and end user acceptance of the use of bioaccessibility values using in vitro methods in human health and ecological risk assessment and policy. Technical Description: The proposed initiative seeks to provide field validated evidence that in vitro bioaccessibility methods can serve as predictive indices of toxic metal bioavailability (in vivo) in DoD soils relative to the more costly and time intensive in vivo feeding studies. By quantifying the extent that soil properties control metal bioavailability, we will show that the predictive models developed in CU-1166 and CU-1210 can be used with a reasonable level of confidence to predict site specific metal bioavailability for DoD soils throughout the United States. By coupling in vitro and in vivo methods at numerous DoD field scale facilities with upfront regulator and end user input, our goal is to obtain regulatory acceptance of in vitro methods and the SBAT tool for assessing toxic metal bioavailability in contaminated DoD soils as it relates to human and ecological risk.
Technology Maturity: Within SERDP CU-1166, a predictive model (SBAT) was developed to assess the relative bioavailability of toxic metals in soils. The model was built on the premise that key soil physical and chemical properties (e.g., Fe-oxide content, organic matter content, pH) were statistically correlated with decreased metal bioaccessibility (as measured by in vitro, PBET technique). Model results were found to be in good agreement with molecular level metal speciation studies and in vivo swine feeding studies (Yang et al., 2002a, 2004). Nevertheless, model validation using in vivo studies on actual DoD field samples is lacking. Such an endeavor is critical if the model is ever to obtain end-user and regulatory acceptance.
In addition, recent publications within our group, investigating the bioavailability of As in soil have found that the in vitro bioaccessibility method (PBET) correlated extremely well with the in vivo method that used non-DoD soils and immature swine as a model for the gastrointestinal function of children (Rodriguez et al., 1999). Similar findings have been reported for soil bound Pb and Cd where the in vitro PBET method correlated very well with in vivo swine feeding studies (Ruby et al., 1996; and Schroder et al., 2003; respectively). Such information has lead to partial regulatory acceptance in England, where the in vitro methods have been used to assess field scale metal bioavailability issues. Our research team are members of the Bioavailability Research Group of Europe where we have established an international collaboration that seeks to demonstrate the appropriateness of in vitro methods for assessing risk associated with soil metal bioavailability. The UK and several countries within the EU have used our data (United States) of coupled in vitro and in vivo soil metal bioavailability to convince the regulatory community, in their respective countries, that in vitro measurements of soil metal bioaccessibility are acceptable estimates of in vivo soil metal bioavailability. However, regulators in the United States remain uncertain that the in vitro methods can adequately predict soil metal bioavailability in humans.
Prior ecotoxicological studies within our group have also been completed that show soil properties similarly affect the bioavailability of As, Cd, Pb, and Zn for soil invertebrates and plants (Lanno and Basta, 2003; SERDP CU-1210). Measures of metal exposure based upon soil extraction techniques, such as chelates (DTPA) and dilute salts (Basta and Gradwohl, 2000; Dayton, 2003), have been coupled with soil chemical and physical properties to develop statistical relationships for estimating metal bioavailability for soil organisms. These statistical models are the first step in the development of models capable of predicting the toxicity of metals to soil invertebrates and plants.
Based on our previous scientific and technical advances in the area of in vitro and in vivo metal bioavailability in soils, we believe that it is timely to apply these techniques to DoD site-specific problems. Such an effort will validate bioaccessibility and bioavailability estimates based on in vitro methods and soil properties for DoD sites. Close corporation with regulators and end users should lead us closer to regulatory acceptance of in vitro methods for assessing toxic metal bioavailability in soils and use of the validated predictive tool SBAT.
Approach and Methodologies: The proposed research will validate the bioavailability screening tool for DoD soils (SBAT from CU 1166; soil extractions from CU 1210) by determining the chemical speciation, bioaccessibility, bioavailability, and toxicity to biological models used to evaluate ecological risk (e.g., plants, earthworms), and human risk (e.g., immature swine model) of metals (Pb, As, Cd, Cr) in soils from DoD sites. Since ingestion is often the primary risk driver at contaminated sites (Exponent, 2001), human risk as related to ingestion will be evaluated rather than dermal pathways. Only four sites are considered for the in vivo swine dosing studies due to the cost of the methods. More soils can be considered as additional funding becomes available. The in vitro ecological models will also be enhanced by considering approximately 10 DoD soils for in vivo ecological bioassay studies (10 contaminated, 10 control), and these soils will be the same as those used for the human-based models in SERDP CU-1166. SERDP CU-1166 screened over 40 DoD soils using the PBET method and these results have served to guide our choice of initial and future DoD sites to consider for additional in vivo studies. This project will also take advantage of the significant prior investment that has been made by SERDP and ESTCP in this area with regards to project CU-1165 and CU-0222, respectively. Both of these projects have similar goals as the proposed work and we plan to collaborate with the PI’s in an effort to leverage the success of our mission. The proposed research strategy will be discussed at a workshop where scientists, regulators, EPA, and end-users will be present in an effort to advance complete or partial acceptance of in vitro methods in human health and ecological risk assessment and policy. The following organizational chart provides an overview of the experimental design of the proposed project (Figure 1.)
An important component of the proposed technical approach is to validate and demonstrate the ability of soil property models (Yang et al., 2002; Stewart et al., 2002) and in vitro techniques to predict metal bioavailability and risk (e.g., ecological, human). Results obtained from methods developed for accessing metal risk-based endpoints for human (CU-1166) and ecological receptors (CU-1210) will be compared with results from well-established standard methods used by risk assessors to determine human risk (U.S. EPA Risk Assessment Guidance for Superfund--RAGS) and ecological risk (U.S. EPA Ecological Risk Assessment) (Figure 2).
Comparison of bioavailability assessment technologies developed by CU-1166 and CU-1210 with bioavailability endpoints in the current U.S. EPA risk assessment framework will satisfy a critical element of the ESTCP proposal requirements to “provide data and support to achieve regulatory and end-user acceptance.” The approach to validate and demonstrate the use of the SBAT tool and in vitro methods to derive risk-based data for end users at the four DoD study sites follows.
Workshops
Immediately upon receiving possible funding for this endeavor, a two day workshop will be held bringing together state regulators, DoD site end users, state EPA officials, and scientists familiar with soil metal bioavailability. A list of potential attendees is presented later in the proposal. We will seek assistance from the Interstate Technology Regulatory Council (ITRC) which is a state-led coalition working together with industry and stakeholders to achieve regulatory acceptance of environmental technologies. ITRC consists of 40 states, the District of Columbia, multiple federal partners, industry participants, and other stakeholders, cooperating to break down barriers and reduce compliance costs, making it easier to use new technologies, and helping states maximize resources. The workshop will focus on past, current, and future research endeavors that are investigating soil metal bioavailability methodologies and the possible use of in vitro bioaccessibility values in human health risk assessment and policy. We will show how data from the United States, and our group in particular, has contributed to the successful regulatory acceptance in England, where the in vitro methods have been used to assess field scale metal bioavailability issues. We will ask the attendees of the workshop to become an integral part of our project by serving as observers and advisors during the course of the research. A State of the Science and Regulatory Acceptance will be developed as a product of the workshop along with the development of the Technology Demonstration Plan. Attendees will be informed that an important outcome of our research is to seek complete or partial acceptance of in vitro methods in human health and ecological risk assessment and policy. Two additional information meetings will be scheduled on a yearly basis as research results become available.
Possible Site Selection
Four (4) DoD facilities with drastically different soil properties, but with a common metal contamination problem with regards to Cr, As, Pb, and Cd will be utilized in the swine dosing trials and ten (10) facilities will be considered in the ecological bioassay studies. Soil types hypothesized to strongly sequester metals will be compared to soil types thought to have poor metal sequestering potential. Example of such DoD sites are Hill AFB – UT, Travis AFB - CA, Deseret Chemical Depot – UT, Aberdeen Proving Ground – MD, and Redstone Arsenal – AL, Naval Station Newport – RI, and Fallon Naval Air Station – NV, all of which have significant problems with metal contaminated soils. Select chemical, physical, and mineralogical, properties of the soils have been quantified in our laboratory as described by Stewart et al. (2002a). Soils at Hill, Deseret, and Fallon Naval Air Station are Aridisols which are sandy, high pH soils with a limited capacity to sequester metals. These soils are expected to have high metal bioaccessibility. Soils from Aberdeen and Travis are silty, neutral pH soils with good to excellent metal sequestering properties. These soils are expected to have low metal bioaccessibility. Redstone and Naval Station Newport are acidic, Fe-oxide rich Ultisols and Inceptisols which has excellent sequestering properties for As, and potentially poor sequestering properties for Cd, Pb, and Cr(VI), thus the latter metals being highly bioaccessible. Reference soil, the same soil series but uncontaminated (i.e., natural background levels of Cd, Pb, As) will also be collected at each of the study sites for each of the contaminated sites.
Each field soil collected will be air-dried and placed in a large plastic tub. Random portions of the soil will then be mixed within a large plastic cement mixer. This subsample is than placed to the side and another random portion of the sample is mixed accordingly. Once all the sample has been mixed, the subsamples are than mixed. The above procedure is repeated five more times resulting in a completely uniform mixed soil. This will be confirmed by total metal analysis of thirty subsamples for each soil type.
Validation/Demonstration of use of SERDP projects CU-1166 and CU-1210 for Human and Ecological Risk Assessment of Metal Contaminated Soil
Soil properties, total metal content, and metal bioaccessibility and bioavailability (as measured by various in vitro and in vivo methods, respectively) will be determined for metal contaminated soils collected from the four DoD sites for the human health models. A similar approach will be taken for the in vitro ecological model and it will be made more robust by considering an additional 20 DoD soils. Three components of method validation and demonstration are:
in vitro methods and soil property models to predict metal bioaccessibility and bioavailability in study soils: Metal bioaccessibility and metal bioavailability for the four study soils will be calculated using soil property-driven models developed from CU-1166 and CU-1210 studies, respectively. Calculated bioaccessibility values will be compared with measured bioaccessibility values using in vitro gastrointestinal methods for study soils. The physiologically based extraction test (PBET) developed by Ruby et al. (1999), will be utilized at a variety of pH conditions to estimate metal bioaccessibility for variety of stomach environments indicative of food intake, or lack thereof. Using the method of Stewart et al. (2002a), additional soil property-driven models will be constructed using the PBET method at these pH values. This is particularly important for Pb contaminated soils since Pb bioaccessibility decreases with an increase in pH (Yang et al., 2002b). In contrast, As(V) bioaccessibility was minimally influenced by changing pH environments.
For ecological risk estimates, metal bioavailability will be estimated from multiple regression and path analysis models developed using toxicity and bioaccumulation data from 26 soils (CU-1210; previous US EPA-NCEA project). Additionally, 10 selected DoD sites (20 soils) from CU-1166 will be tested in addition to the four soils proposed above. This is necessary to enhance to robustness of the ecological model from CU-1210 as has already been done for the human-based model in CU-1166. In the ecological investigations, data from in vitro DoD soil metal extraction coupled with DoD soil chemical and physical properties will be compared to existing statistical relationships for estimating metal bioavailability to plants and soil invertebrates. Initially, statistical relationships developed for metal availability from a set of 26 soils will be used to estimate the chemical availability of metals in DoD soils, based upon total metal levels and soil physical/chemical characteristics. This will be followed by extraction of the DoD soils using several wet chemical methods (e.g., extraction with chelates (DTPA) or dilute salts (Ca(NO3)2; Basta and Gradwohl, 2000; Dayton, 2003) to actually measure the chemical availability of metals in DoD soils. These measurements will be compared to predicted chemical availability estimated by the models to determine the ability of the models to predict metal availability. The statistical models will also be used to predict the toxicity of the DoD soils to earthworms and plants, assuming additivity of the toxicity of individual metals. Bioassays will be conducted with DoD soils to determine actual toxicity and these results will be compared to the model predictions. Comparison of the actual toxicity from bioassays with predicted toxicity from in vitro models will be used to quantify the ability of in vitro models to predict actual ecotoxicity in field DoD soils. This will be the basis for validation of the in vitro methods for field DoD soils.
In an effort to validate the physical significance of the soil property models used to describe the bioaccessibility of metals in the DoD soils, the mechanisms of enhanced metal sequestration and solid-phase metal speciation will be quantified with a variety of high-resolution surface spectroscopy techniques. Such techniques will include Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM-EDS) and X-ray Absorption Spectroscopy (XAS). Bulk SEM-EDS measurements will be conducted with the premier facilities for determining the environmental speciation of metals, located at DOE’s Environmental Molecular Science Laboratory (EMSL), Pacific Northwest Laboratory, Richland, WA., which will provide direct quantification of the mineralogic and nature of solid phase contaminants that are present. These facilities are state-of-the-art with a field emission SEM having resolutions of at least 1.5 nm at 30 KeV and 4.0 nm at 1.0KeV. This technique is useful for determining the crystalline domains of the solids, and with associated energy dispersive spectroscopy, elemental composition. Our research group has extensive experience in the use of this interfacial interrogation technique for monitoring changes in the mineralogy of toxic metals in heterogeneous media and we have a good working relationship with the EMSL staff.
Metal speciation will be assessed on the four DoD soil types using X-ray Absorption Spectroscopy at the Advanced Photon Source (APS) and the Stanford Synchrotron Radiation Laboratory (SSRL). We will elucidate the chemical and structural environment (valence state and surface configuration) of each metal on the various soils using XAS. This effort will provide an improved conceptual understanding of the molecular-level speciation of Pb, Cd, Cr, and As in the soils, and how the molecular speciation influences the resulting bioaccessibility. All of the elements that are the focus of this research have core electron excitation energies between 8 and 26 KeV, making them ideal for synchrotron research. High-intensity synchrotron x-ray sources permit such analysis of undisturbed samples and with new available focused beams allow spatial heterogeneity to be appreciated. XAS is one of the few atomic techniques for obtaining molecular level information that can be conducted in unaltered samples, which is crucial for examining the true in situ molecular-level speciation of these metals. The detection limits for synchrotron-generated XAS vary depending on the matrix, but samples with concentrations greater than 10 mg/kg should yield good results. Our research group has extensive experience in the use of XAS to monitor changes in molecular speciation of toxic metals and radionuclides in heterogeneous media and we have a good working relationship with the SSRL and APS staff. The metal speciation results will be used to confirmed macroscopic observations of metal bioavailability for both the in vitro and in vivo methods (Stewart et al., 2002b, Yang et al., 2004).
Validation/demonstration of in vitro methods to predict metal bioavailability of study soils: Metal bioaccessibility calculated by CU-1166 in vitro methods using more than 40 DoD soils will be correlated with metal bioavailability using in vivo immature swine dosing trials. The pig has been used as an animal model in a number of research fields including gastroenterology, nutrition, and metabolism. Specific justification for the use of swine in chemical bioavailability studies on soil matrices revolves primarily around biological (anatomical, physiological, biochemical) similarities to humans. There is an extensive database of information on the use of the swine model. Standard operating procedures (SOPs) using the immature swine model developed by Dr. Stan Casteel, University of Missouri-Columbia Veterinary Medical Diagnostic Laboratory, have been approved by the USEPA Region 8 (Casteel, 1995) for measuring the bioavailability of Pb from incidental ingestion of soils by children. During the past 10 years, the swine model has served well as a surrogate for study of systemic bioavailability of soil Pb in a sensitive population of humans. More than 30 Superfund Site soils from locations across the nation have been tested. The swine model uses relative bioavailability data as measured by comparing oral absorption of the metal of interest in test soils to oral absorption of some fully soluble form of the metal. The fraction of the absorbed dose of a metal can be measured using concentrations in blood and tissues such as liver, kidney, and bone. For the special case of As, the urinary excretion fraction is most appropriate. It has been shown by Weis et al. that preliminary site-specific estimates of soil Pb relative bioavailability on 20 soils of concern to the USEPA ranged from 6% to greater than 85%, relative to the absorption measured for Pb from lead acetate. The model has also been used successfully to assess the bioavailability of Cd and As.
An example of the general study design for the Pb-contaminated soils dosing trial is as follows where two different contaminated soils are shown with their soluble control at three different dosing levels and five replications:
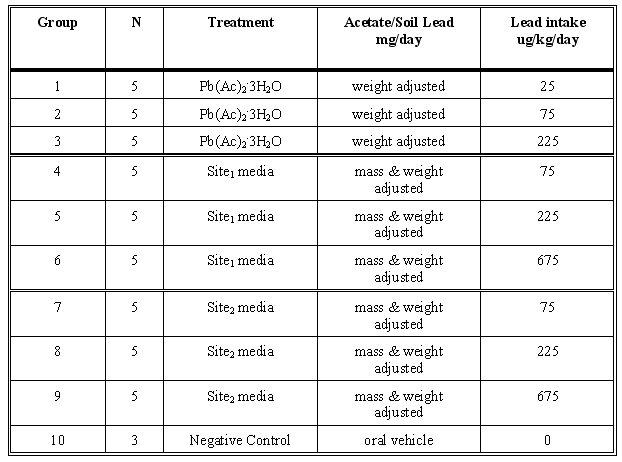
Intact male pigs weighing 10-12 kg will be housed individually in lead-free cages and fed low-lead feed. All doses will be delivered daily for 15 days in a low-lead vehicle according to the diurnal schedule. One blood sample (6-8 mls) will be drawn (following SOP #9) from each animal on days 0, 1, 2, 3, 5, 7, 9, 12, and 15, into a new plastic lead-free syringe by venipuncture of the anterior vena cava. The blood will be immediately transferred into lead-free VacutainerR tubes containing EDTA. In each case, blood samples will be drawn 17 hours after the second dosing of the previous day. Animal weights will be recorded and doses and feed adjusted on days -1, 2 and every third day thereafter until study termination. Blood samples will be prepared as per SOP #11. Animals will be fed according to the regular daily schedule outlined in the Project Notebook. On study day #15, pigs will be humanely sacrificed and representative samples of liver, kidney, and bone will be collected and prepared for analysis as per SOP #11.
Metal availability estimated by models developed in CU-1210 will be compared and correlated with metal bioavailability using plant bioassay and earthworm (e.g., soil invertebrate) bioassays. Metal bioavailability and ecotoxicity in contaminated soils collected from DoD sites will initially be assessed using soil invertebrate bioassays with earthworms (Eisenia fetida), potworms (Enchytraeus albidus), and collembola (Folsomia candida) according to standard protocols. Bioassay endpoints will include mortality, reproduction, and internal concentration of metals (bioaccumulation). Plant bioassays with Alfalfa, Medicago sativa; Perennial ryegrass, Lolium perrene; and Japanese millet, Echinochloa esculenta will be conducted with contaminated soils from DoD to provide plant risk-based endpoints of germination, dry matter growth, and tissue metal concentrations.
The in vivo results will be compared to results from the various soil property-driven models in an effort to show that the cost-effective in vitro methods can serve as a screening tool for estimating toxic metal bioavailability. This information can in turn be used to prioritize DoD sites in terms of their potential ecological risk and the need for more-detailed, and costly, site-specific bioavailability (e.g., animal dosing, plant and invertebrate) studies.
Demonstration and use of in vitro methods to perform human and ecological risk calculations for contaminated study soils: Risk estimates from incidental ingestion of contaminated soils will be calculated using metal bioavailability values derived from CU-1166 methods (total metal content and soil properties). Adjustments to ecological risk-based endpoints (bioaccumulation, ecotoxicity) based upon study soil properties will be calculated using methods developed in CU-1210. As is being done in CU-1350, neural network models will be implemented in a spreadsheet program to compute health risk due to ingestion of one or more metals of interest and any given soil properties. The program will compute confidence limits on risk estimates due to combined effects of intrinsic model uncertainty and to uncertainty in soil properties (e.g., as estimated from tabulated data for various soil types). The results from this task will provide a tool to evaluate risk reduction due to toxic metal sequestration in soils to support DoD’s performance/risk assessment and decision-making process for military base site restoration.
Cost/Benefit of Technology
The results from this project would provide site managers and risk assessors with tools to make better initial estimates of site risk which can be used to prioritize sites and to justify, on basis of cost savings from estimated Environmentally Acceptable Endpoints, more definitive site-specific in vivo bioavailability studies. Measures of metal bioavailability can be used to eliminate sites or portions of sites from further risk assessment procedures during screening or Phase I procedures. Two types of approaches could be used: 1) where background data on the site such as total metal levels and soil properties are available, direct application of the models developed from CU-1166 and CU-1210 would provide estimates of the hazard posed by metals at the site; 2) for sites where little information is available, chemical data such as an in vitro GI extraction for human risk or a weak salt extraction for ecological risk would be meaningful for making a decision regarding the site. These values would be compared to screening criteria to determine whether any further assessment is warranted. These concepts are quite unique in that site risks are based on bioavailability estimates versus the current standard of basing site risk on traditional total soil metal analyses; concepts that could save DoD huge expenses in unnecessary remedial costs.
Soil excavation and landfilling costs have been estimated at US $730 m-2 to a 60 cm depth (Vangronsveld and Cunningham, 1998). Remediation costs associated with soil excavation and replacement exceeding $10,000,000 per site are not uncommon. Many times, excavation is performed because risk assessments assume the contaminant is highly bioavailable (i.e., 60% for Pb, 100% for As, Cd, and Cr). Use of in vitro methods to assess contaminant bioavailability will identify soils that have low contaminant bioavailability and/or little/acceptable risk and not require remediation via excavation/replacement. in vitro methods will help focus prudent use of limited fiscal resources for contaminant remediation and cleanup on DoD sites. Regulatory acceptance of in vitro methods will produce cost savings in the range of billions of dollars.
Technology Transition
The transition of this technology to the end-users requires an integrated effort with regulatory agencies to get buy in. With the help of the Interstate Technology Regulatory Council (ITRC), the project team will be forming a regulatory advisory board to participate in all three stages of the project. The regulatory advisory board will be made up of between five and ten individuals from state, and federal regulatory agencies (contacts have been made for this advisory board with individuals from the Department of Toxic Substances Control in California and the EPA). All site demonstrations will be focused on end-user needs, and data gathered will be used by DoD project managers and regulatory agencies to make risk based decisions at the demonstration site. The other requirement for technology transition is to disseminate information about this technology to DoD project managers and their contractors. This will be accomplished by presentation at conferences including the SERDP/ESTCP symposium. Other potential conference presentations include the Society of Environmental Toxicology and Chemistry (SETAC) and the Society for Risk Analysis (SRA). It will also be accomplished by publication of articles in the Navy’s Currents magazine, other similar DoD publications, and external peer-reviewed publications. Our team is unmatched with regard to peer-reviewed publications and dissemination of information to the scientific community.
Performers
This project will involve the collaborative efforts of the following performers: Amy Hawkins, Naval Facilities Engineering Service Center will coordinate regulatory and end-user involvement; Dr. Philip Jardine, Oak Ridge National Laboratory will lead the effort for the demonstration and validation of models to predict bioaccesibility; Dr. Roman Lanno and Dr. Nick Basta, Ohio State University will be responsible for in vivo ecological bioassays; Dr. Stan Casteel, University of Missouri, Columbia will do in vivo swine dosing trials for model validation; and Dr. Scott Fendorf, Stanford University will provide metals speciation for use in the models.
Amy Hawkins is a biologist in the consultation/information management branch of the Naval Facilities Engineering Service Center (NFESC). As a member of the Ecological Risk Technical Assistance Team (ERTAT) her duties include providing review of ecological risk assessments, site-specific application of Navy policy, and management of risk assessment-related research and development projects. She has provided ecological risk assessment technical support at more than 30 Navy sites. She has presented various technologies as part of NAVFAC’s Remedial Innovative Technology Seminars (RITS) and now serves on the technical review team for the RITS. Ms. Hawkins has also managed various projects implementing new technologies through the Navy’s Broad Agency Announcement.
Dr. Philip Jardine is a Distinguished Research Staff Scientist at ORNL. He specializes in subsurface science research that deals with time-dependent, multi-process fate and transport issues at multiple scales. His current research activities include chemical and microbial controls on contaminant fate and transport, experimental and theoretical aspects of subsurface contaminant migration at laboratory and field scales, quantifying the bioavailability of toxic metals in contaminated soils. Twelve national and international scientific awards. Over 130 peer-reviewed publications.
Website: http://www.esd.ornl.gov/earth_aquatic/people/p_jardine.shtml
Dr. Roman Lanno is an Associate Professor of Entomology at Ohio State University. He has over 18 years experience in ecotoxicology with research the last seven years focusing on issues of chemical bioavailability, toxicity, and bioaccumulation or organic chemicals and metals in soil invertebrates, specifically using the earthworm as a model. His bioavailability research has involved examining abiotic and biotic modifying factors in determining the bioavailability of chemicals via dermal and intestinal routes of exposure. He has been invited to give presentations on the bioavailability and toxicity of chemicals in the environment by such agencies as the US EPA (Metals Bioavailability White Paper), National Environmental Policy Institute (NEPI), and the United Nations European Council on the Economy (UN ECE). He has participated in a number of SETAC Pellston Workshops in soil and aquatic toxicology and is editor of “Assessing Contaminated Soils: From Soil-Contaminant Interactions to Ecosystem Management”, the publication resulting from one of these workshops.
Dr. Nick Basta is Associate Professor of Soil and Environmental Chemistry in the School of Natural Resources at The Ohio State University. Dr. Basta has active research and instruction programs that focus on environmental soil chemistry including the risk-based environmental chemistry of organic and inorganic pollutants in contaminated soils with emphasis on bioavailability and contaminant transmission to human and ecological receptors. He has authored or co-authored > 50 manuscripts in refereed journals, 7 book chapters, and 130 abstracts and proceedings of presentations at scientific meetings. Co-Chair, CSREES Technical Committee, "Chemistry and Bioavailability of Waste Constituents in Soils," International Society for Trace Element Biogeochemistry, Steering Committee, and Contaminated Soil Advisory Group, Society for Environmental Toxicology and Chemistry.
Dr. Stan Casteel: Professor and Director of the Veterinary Medical Diagnostic Laboratory at the University of Missouri’s College of Veterinary Medicine. Research Background: Solving animal disease problems, teaching veterinary students and doing research in environmental risk assessment represent the body of his work. As a diagnostician and researcher Dr. Casteel has given more than 150 presentations at scientific meetings and has authored more than 150 abstracts and scientific papers and 29 book chapters. Major funding and current research efforts focus on an understanding of the biokinetics of lead, arsenic and cadmium from contaminated industrial matrices using juvenile and adult-pregnant swine as models for children and pregnant women. This effort is specifically directed toward improving the understanding of the absorption of arsenic, lead and cadmium from contaminated media from various EPA Superfund sites, many of which are on the National Priority List. This is an important departure from EPA's default assumptions regarding heavy metal bioavailability. The impetus for this departure is to provide additional scientific evidence in support of EPA's integrated exposure uptake biokinetic model and site-specific data generated from Superfund Site test soils.
Website: http://www.cvm.missouri.edu/vpbio/faculty/casteel.html
Dr. Scott Fendorf is an Associate Professor at Stanford University. Research Background: Interfacial chemical processes that influence the partitioning , and hence bioavailability, of metals between solution and solid phase. Use of novel, high-resolution surface spectroscopy techniques to decipher the speciation and chemical environment of metals on solid surfaces. 4 national scientific research awards. Over 55 articles published in peer-reviewed journals.
Website: http://soils.stanford.edu/new/Members/members1.asp
Mark O. Barnett is an associate professor at Auburn University. Research background: soil property controls on metal bioavailability in soils; speciation, transformation, and transport of toxic metals in the subsurface. Notable: 10+ years environmental engineering experience at DoD and DOE facilities, including key project scientist for remediation of Hg-contaminated Superfund site in Oak Ridge, TN. Speciation and bioavailability results were adopted resulting in millions of dollars in cost savings. Work cited by EPA Regional Administrator and awarded ORNL Corp. President’s Award (1995). Over 20 peer-reviewed journal articles.
References
Basta, N.T., and R. Gradwohl. 2000. Estimation of Cd, Pb, and Zn bioavailability in smelter-contaminated soils by a sequential extraction procedure. J. Soil Contam. 9:149-164.
Casteel S.W., R.P. Cowart, C.P Weis et al: 1997. Bioavailability of Lead to Juvenile Swine Dosed with Soil From the Smuggler Mountain NPL Site of Aspen, Colorado. Fundam. Appl. Toxicol. 36:177-187.
Dayton, E.A. 2003. Relative contribution of soil properties to modifying the phytotoxicity and bioaccumulation of cadmium, lead and zinc to lettuce. Ph.D. Dissertation, Oklahoma State University, Stillwater, OK.
Exponent, 2001. White Paper: Evaluation of the Metals that Drive Risk-Based Remedial Decisions at DoD Sites. Prepared for SERDP, September 2001.
Heuscher, S.A., C.C. Brandt, and P.M. Jardine. 2004. SBAT: A Tool for Estimating Metal Bioavailability in Soils. Oak Ridge National Laboratory ORNL/TM-2004/49.
Lanno, R.P., and N.T. Basta. 2003. An integrated chemical and toxicological approach of evaluating the chemical and biological availability of metals in soil. U.S. Environmental Protection Agency National Center for Environmental Research. EPA Grant Number CR 827230-01-0. Final Report. 129pp.
Rodriguez, R. R., N. T. Basta, S. W. Casteel and L. W. Pace (1999). “An in vitro gastrointestinal method to estimate bioavailable arsenic in contaminated soils and solid media.” Environmental Science & Technology 33(4): 642-649.
Ruby, M. V., A. Davis, R. Schoof, S. Eberle and C. M. Sellstone (1996). “Estimation of lead and arsenic bioavailability using a physiologically based extraction test.” Environmental Science and Technology 30(2): 422-430.
Schroder, J.L., N.T. Basta, J. Si, S.W. Castell, T. Evans, and M. Payton. 2003. Environ. Sci. Technol. 37:1365-1370.
Stewart, M.A., P.M. Jardine, M.O. Barnett, T.L. Mehlhorn, K. Hyder, and L. McKay. 2003. Influence of soil geochemical and physical properties on the sorption and bioaccessability of Cr(III). J. Environ. Qual. 32:129-137.
Stewart, M.A., P.M. Jardine, C.C. Brandt, M.O. Barnett, S.E. Fendorf, L.D. McKay, T.L. Mehlhorn, and K. Paul. 2003. Effects of contaminant concentration, aging, and soil properties on the bioaccessibility of Cr(III) and Cr(VI) in contaminanted soils. Soil and Sediment Contamination 12:1-21.
Vangronsveld, J., and S.D. Cunningham. 1998. Introduction to the concepts. In J. Vangronsveld and S.D. Cunningham (eds.) Metal-contaminated soils: In situ inactivation and phytorestoration. Springer-Verlag, Berlin.
Yang, J. K., M. O. Barnett, S.E. Fendorf, and P. M. Jardine. “Oxidation and bioaccessibility of As(III) in soils.” Environ. Sci. Technol. (submitted).
Yang, J.K., M.O. Barnett, P.M. Jardine, and S.C. Brooks. 2003. Factors controlling the bioaccessibility of arsenic(V) and lead(II) in soil. Soil and Sediment Contamination. 12(2):165-179.
Yang, J.-K., M.O. Barnett, P.M. Jardine, N.T. Basta, and S.W. Casteel. 2002. Adsorption, sequestration, and bioaccessibility of As(V) in soils. Environ. Sci. Technol. 36:4562-4569.
For more information, contact:
Philip Jardine (jardinepm@ornl.gov, 865-574-8058)

