Perchlorate Treatment – Using Highly Selective, Regenerable Ion Exchange Resins
News: ORNL System Eliminates Perchlorate, Helps Scientists Trace Source
Book: Perchlorate Environmental Occurrence, Interactions and Treatment
 |
|
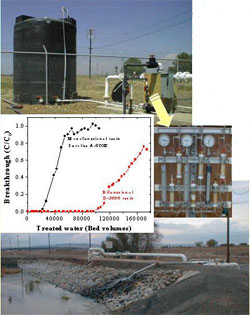
Perchlorate (ClO4-) has emerged as a significant new threat to groundwater and drinking water supplies and the environment. The Oak Ridge National laboratory recently developed a new class of bifunctional anion exchange resins, which are highly selective for sorption of ClO4- from contaminated groundwater or surface water. A field experiment demonstrated that the bifunctional resin was able to treat >100,000 bed volumes of groundwater before a 10% breakthrough of ClO4- occurred (running at ~2 bed volumes per minute with an initial ClO4- concentration of ~50 mg/L). The bifunctional resins are particularly effective in removing trace quantities of ClO4- in groundwater to below the detection limit (~1 mg/L). Using pertechnetate (TcO4-) as an analog (with similar chemical properties as ClO4-), these bifunctional resins were able to remove TcO4- from contaminated groundwater at below 0.001-ppb levels. No pretreatment is needed to remove either dissolved organic matter or other competing anions (such as Cl-, SO4=, HCO3-, or NO3-), which may be present at 3–5 orders of magnitude higher than that of ClO4- in the groundwater or surface water. The treatment process does not involve addition or removal of unwanted organic or inorganic compounds or nutrients in the water because of the high selectivity of the bifunctional resins.
Additionally, a new resin regeneration technology was developed for regenerating the bifunctional and other selective anion-exchange resins (e.g., Purolite A-520E) using the ferric chloride-HCl displacement technique (US patents: 6,448,299, 6,358,396). Laboratory experiments indicated that a nearly 100% recovery of ion-exchange sites was achieved by washing with as little as ~2 bed volumes of the ferric chloride regenerant solution in a column flow-through system. There was no significant deterioration of the resin’s performance with respect to ClO4- removal after repeated loading and regeneration cycles.
Another innovative technology was recently developed for a complete destruction of perchlorate in the ferric chloride regenerant solutions (US patent pending, 10/157,407). While perchlorate is destroyed, the treatment process does not alter the properties of the regenerant solution so that it can be used repeatedly and no waste regenerant is produced. This treatment process therefore has enormous economic implications that not only a reduced volume of regenerant is required but also the disposal of hazardous wastes containing perchlorate is eliminated. The treatment process is efficient and cost-effective, while not being subject to difficult–to–maintain operating conditions.
Conventional Ion Exchange |
Highly-Selective Resins |
|
|
Potential In-Situ or Ex-Situ Applications
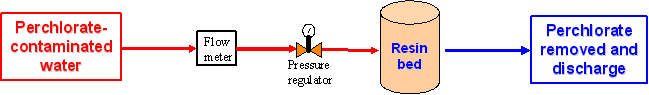
1. Illustration of Ex-situ Water Treatment at Wellheads
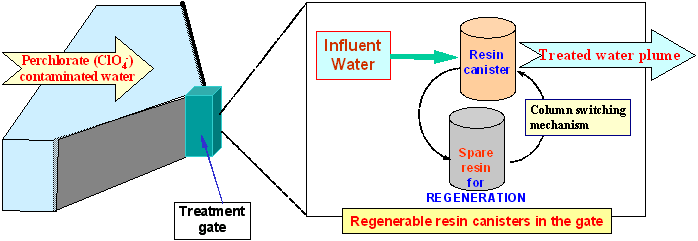
2. Illustration of In-situ Funnel-and-Gate Treatment Systems
For more information, contact:
Baohua Gu (gub1@ornl.gov, 865-574-7286)

