Biogeochemical Transformations at Critical Interfaces
ORNL Mercury Science Focus Area (SFA)
Theme 1: Ecosystem Features Influencing Mercury Transformation
Theme 1 research examines the biogeochemical controls on Hg methylation and demethylation within the context of the flowing creek system and its connection with the surrounding watershed. Emphasis is on field-based investigations with supporting laboratory work to elucidate mechanisms.
There are three overarching objectives:
- Identify ecosystem domains and hydrobiogeochemical conditions that govern net MeHg concentrations in EFPC.
- Identify microbial community traits and interactions that are important within periphyton and sediment ecosystems that influence Hg transformations and storage.
- Work iteratively with ongoing modeling activities to inform and support the biogeochemical modeling framework.
These objectives are addressed through a set of hypotheses-driven field and laboratory investigations and the development of a process-rich numerical model to challenge current understanding of watershed processes occurring over broad spatiotemporal scales.
FY20–FY21 Accomplishments
Over the past 12 months, Theme 1 made significant progress toward milestones and published several papers relating to the role of periphyton in Hg cycling and development of predictive models of those reactions. Additional papers have been published reporting on the effect of Hg(II) sorption on MeHg production and on improvements to predicting the equilibrium aqueous speciation of Hg.
Role of Periphyton in EFPC Mercury Cycling
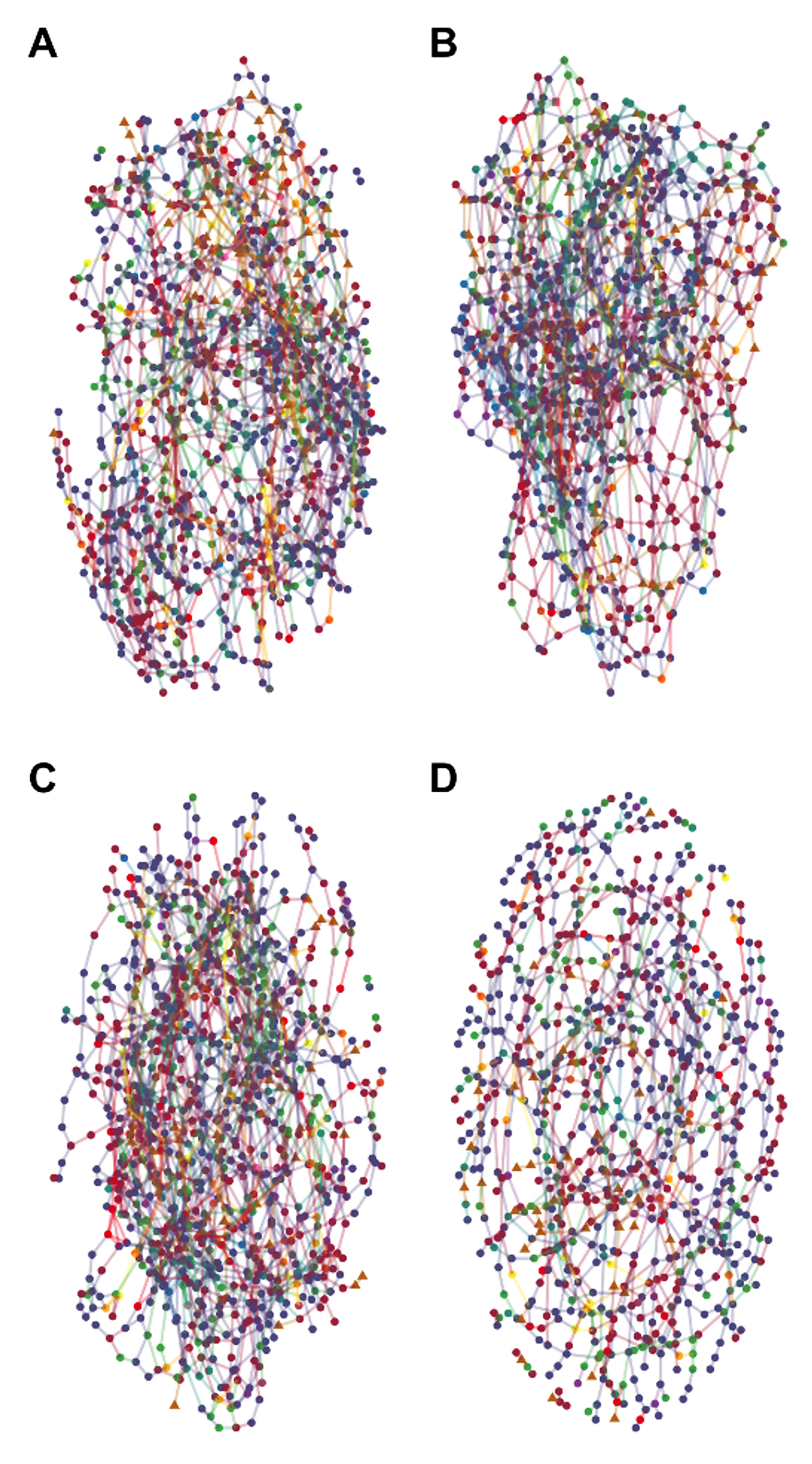
click to enlarge
Cross-Domain Correlation Networks. Charts show correlation networks of archaea, bacteria, and fungal taxa for (A) control, (B) nitrate, (C) phosphate, and (D) nitrate + phosphate nutrient exposures. Mercury methylation was highest in control and nitrate samples, suggesting these well-connected hubs may explain higher methylation.
Using high-throughput amplicon sequencing of the 16S rRNA gene, ITS2 region, and Hg-methylation gene pair (hgcAB), we characterized the archaea, bacteria, fungi, and Hg-methylating microorganisms in periphyton communities in the stream corridor of the EFPC watershed. Further, we examined how nutrient amendments (nitrate and phosphate) altered periphyton community structure and function. Overall, we found that Hg-methylation potential correlated with numerous bacterial families that do not contain hgcAB, suggesting that overall microbiome structure of periphyton communities influence rates of Hg transformation. Interestingly, the addition of nitrate to these habitats resulted in the most connected periphyton microbial communities, which correlated with enhanced Hg-methylation potential. This research provides insight into community interactions within the periphyton microbiome that may contribute to Hg cycling and will inform future research that will focus on establishing mixed microbial consortia to uncover mechanisms driving shifts in Hg cycling within periphyton habitats (Carrell et al. 2021).
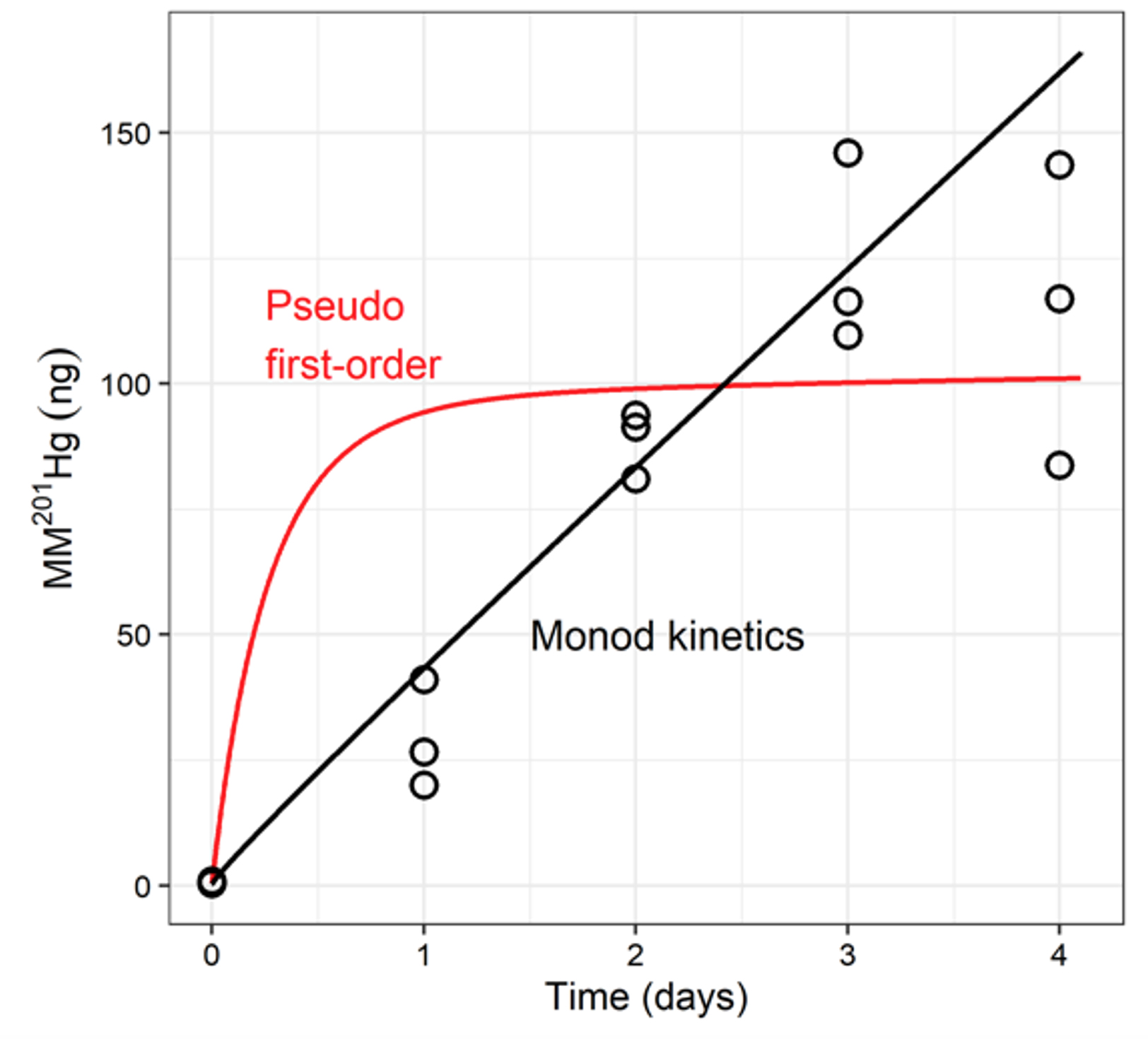
click to enlarge
Transient Availability Model (TAM). Production of MM201Hg over time in sediment microcosm experiments. The lines indicate the TAM using either pseudo first-order (red) or Monod (black) kinetic expressions of MMHg production.
In anoxic environments, anaerobic microorganisms carrying the hgcAB gene cluster can mediate Hg transformation to monomethylmercury (MMHg). The kinetics of Hg transformation to MMHg in periphyton from EFPC have previously been modeled using a transient availability model (TAM). The TAM for Hg methylation combines kinetic expressions for processes that reduce Hg and MMHg availability for methylation and demethylation (multisite sorption of Hg and MMHg, Hg(II) reduction/ Hg(0) oxidation) with methylation/demethylation kinetics. In this study, the TAM is used for the first time to describe MMHg production in sediment. We assessed MMHg production in sediment microcosms using two different sediment types from EFPC: a carbon-rich sediment with lower, more anoxic redox potential and a sandy, carbon-poor sediment with a higher redox potential. Based on 16s rRNA sequencing, the overall microbial community structure in the two sediments was retained during the incubations. However, the hgcA containing methanogenic Euryarchaeota communities differed between sediment types, and their growth followed different trajectories over the course of incubations, potentially contributing to the distinct patterns of MMHg production observed. The general TAM paradigm performed well in describing MMHg production in the sediments. However, the MMHg production and ancillary data suggested the need to revise the model structure to incorporate terms for variable microbial activity. We modified the TAM to include Monod-type kinetics for methylation and demethylation and observed an improved fit for the carbon-rich, microbially active sediment . Overall, our work shows that the TAM can be applied to describe Hg methylation in sediments. In some cases, including expressions that account for variable microbial activity can improve the accuracy of the model description of the data.
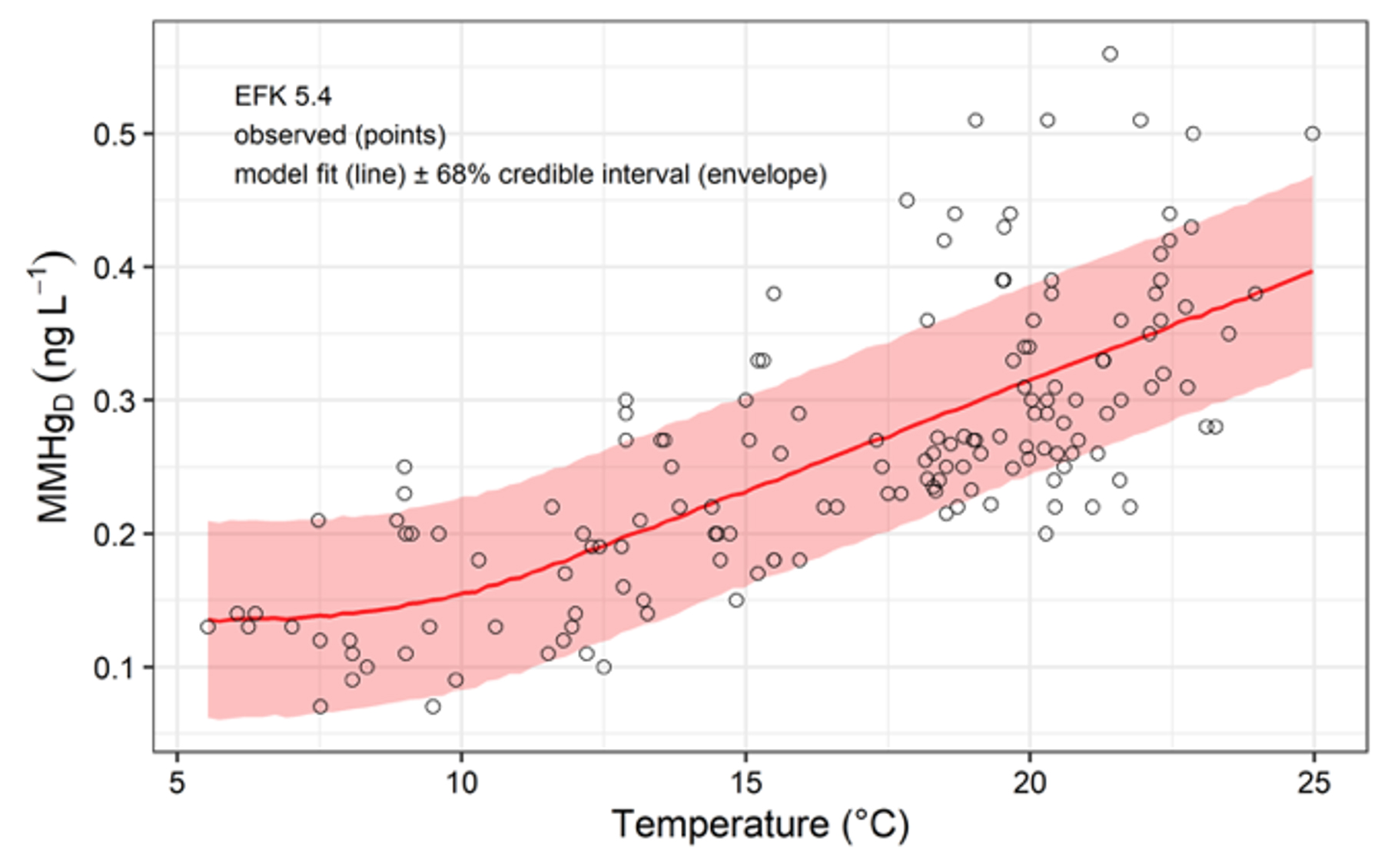
click to enlarge
Dissolved MMHg Concentration vs. Water Temperature. MMHg concentration is low, relatively constant at ~0.13 ng/L. It is independent of temperature below 10°C and significantly positively correlated with temperature above that threshold.
EFPC stream restoration activities included the initiation of a flow management program in 1996 in which water from a nearby lake was pumped to the head of the creek. We conducted regular water sampling for two years along the length of EFPC during active flow management and for five years after flow management stopped. Total Hg and total MMHg concentration and flux decreased in the uppermost reaches of EFPC that were closest to the point of water addition. Most water quality parameters, including dissolved organic carbon (DOC) concentration, remained unchanged after flow management termination. Nevertheless, SUVA254, a measure of dissolved organic matter (DOM) composition, increased and coincided with increased dissolved Hg (HgD) concentration and flux and decreased Hg solid-water partitioning coefficients throughout EFPC. Higher SUVA254 and HgD concentration have potential implications for bioavailability and MMHg production. Total and dissolved MMHg concentrations increased in lower reaches of EFPC after the end of flow management, and these increases were most pronounced during spring and early summer when biota are more susceptible to exposure and uptake. After active flow management ended, a general warming trend in the creek likely acted in concert with higher HgD concentration to promote higher MMHg concentration. Total and dissolved MMHg concentrations were positively correlated with water temperature above a threshold value of 10°C. Concentration changes for Hg and MMHg could not be accounted for by changes in creek discharge that accompanied the cessation of flow management. In addition to the changing DOM composition in-stream, other watershed-scale factors likely contributed to the observed patterns, as these changes occurred over months, rather than instantaneously, after flow management stopped. Nevertheless, similar changes in MMHg have not been observed in a tributary to EFPC.
References Cited
Carrell, A. A., G. E. Schwartz, M. A. Cregger, C. M. Gionfriddo, D. A. Elias, R. L. Wilpiszeski, D. M. Klingeman, A. M. Wymore, K. A. Muller, and S. C. Brooks. 2021. “Nutrient
exposure alters microbial composition, structure, and mercury methylating activity in periphyton in a contaminated watershed.” Frontiers in Microbiology. 12:543(647861). DOI:10.3389/fmicb.2021.647861.
 ORNL Mercury SFA sponsored by Subsurface Biogeochemical Research (SBR) program, U.S. Department of Energy's Office of Biological and Environmental Research. Paul Bayer, SBR Program Manager.
ORNL Mercury SFA sponsored by Subsurface Biogeochemical Research (SBR) program, U.S. Department of Energy's Office of Biological and Environmental Research. Paul Bayer, SBR Program Manager.
Security Notice | Contact Eric Pierce, ORNL | Website Questions | Site Map
File last modified: Wednesday, September 01, 2021
The ORNL Mercury SFA is sponsored by the Subsurface Biogeochemical Research (SBR) program within the U.S. Department of Energy's Office of Biological and Environmental Research.