Mineralogical Characteristics
 |
  |
| Core sampling | An iron core and minerals |
Iron corrosion in groundwater results in the formation of ferrous or ferric ions (when dissolved O2 is present), which ultimately form iron oxyhydroxide mineral precipitates because of their low solubility. It is not surprising, therefore, that many investigators observed iron oxyhydroxides to be the predominant minerals found in the iron reactive barriers (Gu et al., 1999; Phillips et al., 2000; Pratt et al., 1997; Roh et al., 2000) . Core samples were taken ~1.2 and 2.5 years after the Fe0 barrier was installed, and X-ray diffraction (XRD) analysis revealed akaganeite (b-FeOOH) as the major iron mineral precipitant throughout the cores, while goethite (a-FeOOH) was present to a lesser extent (Phillips et al., 2000) . Although they were not detected by the XRD analysis, amorphous iron oxyhydroxide deposits were also observed throughout the iron core materials by means of scanning electron microscope (SEM) and energy dispersive X-ray (EDX) spectroscopic analyses. Presumably, these amorphous iron oxyhydroxides gradually transform to crystalline akaganeite and goethite within the barrier. The formation of akaganeite may be related to a relatively high concentration of chloride in groundwater entering the trench, because, in laboratory studies, akaganeite is commonly observed as the dominant mineral phase from precipitation of ferric chloride (Schwertmann and Cornell, 1991) . The presence of goethite within the Fe0 barrier instead of lepidocrocite, which has been reported in laboratory column studies (Gu et al., 1999) , could result from relatively high dissolved O2 and bicarbonate contents of the groundwater. It has been reported that the formation of goethite is favored over the formation of lepidocrocite when carbonates or CO2 are present in the system (Schwertmann and Taylor, 1977) .
Although to a lesser extent, green rusts were also observed as corrosion products in the Fe0 barrier, and similar observations have been reported previously (Gu et al., 1999; Phillips et al., 2000; Roh et al., 2000) . However, green rusts are not stable and can transform into crystalline iron minerals quickly when exposed to the air or subjected to oven drying. Therefore, care must be taken in sample preservation and preparation in order to observe green rusts in the iron barrier material (Phillips et al., 2001) .
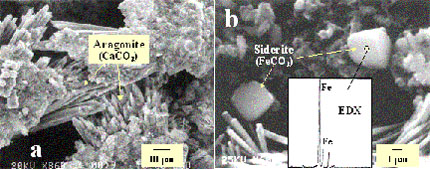
In addition to iron oxyhydroxide minerals, analysis of Fe0 core materials indicated the presence of abundant calcium carbonates such as aragonite (CaCO3) and siderite (FeCO3) (Figures above). These results are consistent with decreased concentrations of calcium and carbonates in groundwater within and downgradient of the Fe0 barrier. Crystalline aragonite was observed throughout the core materials of the Fe0 barrier, and its structure and forms were identified by both SEM and EDX analyses . As indicated previously, relatively high concentrations of Ca2+ and carbonates, coupled with a relatively high pH within the Fe0 barrier, may be largely responsible for the precipitation of CaCO3 minerals (Phillips et al., 2000) .
The formation of ferrous carbonate (i.e., siderite) offers another mechanism for a decreased carbonate or bicarbonate concentration in the Fe0 barrier. Ferrous iron is one of the major byproducts of Fe0 corrosion in groundwater; it is thus conceivable that the formation of siderite can be a favorable reaction when high amounts of carbonate are present in groundwater, particularly at a relatively high pH condition (Mackenzie et al., 1999) . The bicarbonate contents from monitoring wells such as TMW-9 were particularly low and could be attributed largely to its precipitation with both Ca2+ and Fe2+ to form carbonate minerals. However, siderite precipitation was much less extensive than aragonite precipitation. The presence of siderite was detected only in patches in some of the iron core samples. Several factors may contribute to these observations. A relatively high pH and high carbonate but low Ca2+ concentrations favor the formation of siderite (Phillips et al., 2000) . On the other hand, a relatively low pH (about neutral) and a low carbonate concentration shift the chemical equilibrium in favor of Fe(OH)2 precipitation. High Ca2+ concentrations in groundwater may compete with Fe2+ for carbonate and form CaCO3 minerals as described above.
Precipitation of amorphous ferrous sulfide ( FeS ) was also detected by SEM-EDX in most of the core samples from the Fe0 barrier (Figure 1). The morphology of FeS appeared to be rounded or bytrodial, and it was commonly observed as coatings on Fe0 filings or other mineral deposits on iron surfaces. Many Fe0 particles were completely encrusted in FeS , and these coated Fe0 filings remained black (rather than rusty) after drying (by vacuum rinsing with acetone), especially those core materials obtained near the interface where groundwater enters the Fe0 barrier (Phillips et al., 2000) . Note that, although there appears to be a high occurrence of FeS , crystalline pyrite was not detected by XRD, perhaps because of the non-crystallinity of the FeS structure. As stated previously, FeS formation in the Fe0 barrier may be largely attributed to the reduction of SO42- to S2- by sulfate-reducing bacteria under a highly reducing environment in the Fe0 barrier. Similar observations also have been reported in both laboratory simulated iron columns and field experiments with a relatively high influent sulfate concentration (Gu et al., 1999; Phillips et al., 2000) . On the other hand, although reduction of SO42- was observed in the Fe0 barriers at the U.S. Coast Guard Support Center at Elizabeth city, NC, and at an industrial facility in upstate New York (Puls et al., 1999; Vogan et al., 1999) , no appreciable amounts of FeS precipitates were observed. These observations may be related to a relatively low SO42- concentration (<20 mg/L) present in the groundwaters at these sites.
Implications for Long-Term Performance
The occurrence of a suite of mineral precipitates could have serious implications for the long-term performance of Fe0 reactive barriers. Specifically, these mineral precipitates commonly exist as coating and cementing materials on Fe0 surfaces. They not only reduce the reactivity of Fe0 and thus its capacity to degrade or retain target contaminants of concern, but also cause the cementation and clogging of the reactive Fe0 filings. Ultimately, they may result in reduced hydraulic conductivity or the diversion of groundwater through the barrier. However, site groundwater geochemistry and contaminant concentration may determine the rate and forms of mineral precipitant formation and thus the life span of the Fe0 permeable reactive barriers. For groundwater of relatively low ionic strength, McMahon et al. (1999) estimated a 0.35% yearly loss of total porosity in the iron-reactive media at the Denver Federal Center site. On the other hand, a fast corrosion rate and mineral precipitant formation have been observed at the Oak Ridge Y-12 barrier site, where groundwater contains relatively high concentrations of bicarbonate, sulfate, nitrate, calcium, and magnesium. Isolated spots of clogged and/or cemented Fe0 media were observed only ~1.2 years after the installation of the Fe0 barrier at the Oak Ridge site (Phillips et al., 2000) .
Yet more extensive cementation and clogging of iron-reactive media were found in the iron core materials taken ~2.5 years after the installation of the Fe0 barrier, particularly at the soil/barrier interfaces where groundwater enters the Fe0 barrier. The cemented iron cores appeared to be hard to break (Figure 2), and a close examination (by SEM) revealed an extensive iron corrosion and subsequent mineral precipitation on Fe0 surfaces (Figure 18b). The SEM-EDX analysis of a polished cross-section of the cemented iron filings indicated that iron oxyhydroxides were the primary mineral precipitates accumulated on or between individual iron particles as a thick rind (Phillips et al., 2000) . These iron oxyhydroxides may therefore be largely responsible for the cementation of Fe0 particles in the barrier. Similarly, Mackenzie et al. (1999) reported the portion of an iron column clogged with iron oxyhydroxides to be a hardened solid mass that greatly decreased hydraulic conductivity. Additionally, the precipitation and formation of aragonite and FeS minerals were also at least partially responsible for binding iron particles in cemented iron core samples (Figure 2). In particular, FeS precipitates exist primarily in the form of coatings on iron surfaces and thus may act as active binding agents as well. It is interesting to note the sequence of mineral precipitation on iron surfaces as viewed by SEM of the polished sections. Iron oxyhydroxides appeared to precipitate first as a corrosion product on Fe0 surfaces. The formation of aragonite followed in response to an increased groundwater pH as a result of iron corrosion, and FeS precipitates then followed as coatings or void fillers on aragonite and iron oxyhydroxide surfaces. The initial delay in the formation of FeS compared with the other precipitates could perhaps be due to the greater length of time needed for an accumulation of a microbial population to facilitate sulfate reduction in the barrier (Gu et al., 1999; Phillips et al., 2000) .
Based on an average Fe0 filing thickness of ~0.5 to 1.25 mm, Phillips et al. (2000) estimated that these Fe0 filings could be completely corroded within ~5 to <10 years under the specific site geochemical conditions. This estimated life span of an iron reactive barrier is substantially shorter than the life spans that have been estimated previously, ~15 to 30 years (Gillham et al., 1994; Liang et al., 2000; McMahon et al., 1999) , and may be explained by the fact that the site groundwater contains relatively high levels of NO3- and HCO3-, both of which are known to accelerate the corrosion of Fe0 (Davies and Burstein, 1980; Gu et al., 1999; Huang et al., 1998) . It is also important to note that mineral precipitation and iron cementation appeared to occur progressively with time. Within ~1.5 years, spotted cementations of Fe0 filings were observed mostly at the interface where groundwater enters the Fe0 barrier. Cementation extended and further developed downgradient of the Fe0 barrier, as observed in the second coring event (~2.5 years after the barrier was installed). An important implication of these observations is that such an uneven distribution of iron corrosion and mineral precipitation could potentially result in early system clogging at the interface regions and therefore shorten the functional lifetime of in situ Fe0 barriers. Therefore, close attention should be given to areas in the barrier that seem more vulnerable to corrosion, mineral precipitation, and subsequent cementation (e.g., where groundwater first enters the barriers). Particular attention should also be given to the geochemical composition and concentration in groundwater, which may largely determine the corrosion rate and thus the life span of the Fe0 reactive barriers.

